Patients treated with TEGSEDI® (inotersen) saw significant benefit in neuropathy and QoL vs placebo1
Mechanism of action
TEGSEDI targets the polyneuropathy of hereditary ATTR amyloidosis at its source1,2
TEGSEDI binds to and causes degradation of TTR mRNA in the liver, inhibiting TTR protein synthesis1
-
Transcription of TTR mRNA
The TTR gene is transcribed into mRNA that encodes the template for TTR production.1
-
TEGSEDI binds to TTR mRNA
TEGSEDI is a highly selective, single-stranded antisense oligonucleotide that can directly target TTR mRNA.1
-
Translation inhibited
The translation process is inhibited, resulting in a reduction of serum TTR protein (median range: 75% to 79%) and TTR protein deposits in tissues.1
Abbreviations: ATTR, transthyretin-mediated amyloidosis; hATTR-PN, hereditary transthyretin-mediated amyloidosis with polyneuropathy; mRNA, messenger RNA; QoL, quality of life; TTR, transthyretin.
TEGSEDI inhibits the synthesis of TTR protein in the liver through degradation of TTR mRNA1
Study designs
TEGSEDI was studied in a robust pivotal study and open-label extension study in adults with the polyneuropathy of hereditary ATTR amyloidosis1,3
- NEURO-TTR was a phase 2/3, randomized, double-blind, placebo-controlled, multicenter clinical trial to assess TEGSEDI in both neuropathy and quality of life2,4
- 97% (135/139) of patients who completed NEURO-TTR elected to continue on to the open-label extension study3

Coprimary endpoints
Patients treated with TEGSEDI saw significant benefit in neuropathy and QoL vs placebo1
mNIS+7 results at 66 weeks
Patients treated with TEGSEDI achieved a 19.7-point benefit in mNIS+7 at 66 weeks vs patients receiving placebo1,2
Abbreviation: SEM, standard error of the mean.
- Baseline mNIS+7 was2
- 79.2 ± 37.0 in the TEGSEDI group (n=112)
- 74.8 ± 39.0 in the placebo group (n=60)
- 77.6 ± 37.6 total (n=172)
37% of patients treated with TEGSEDI saw improvement at 66 weeks vs 19% of patients receiving placebo (P=0.033)2,4
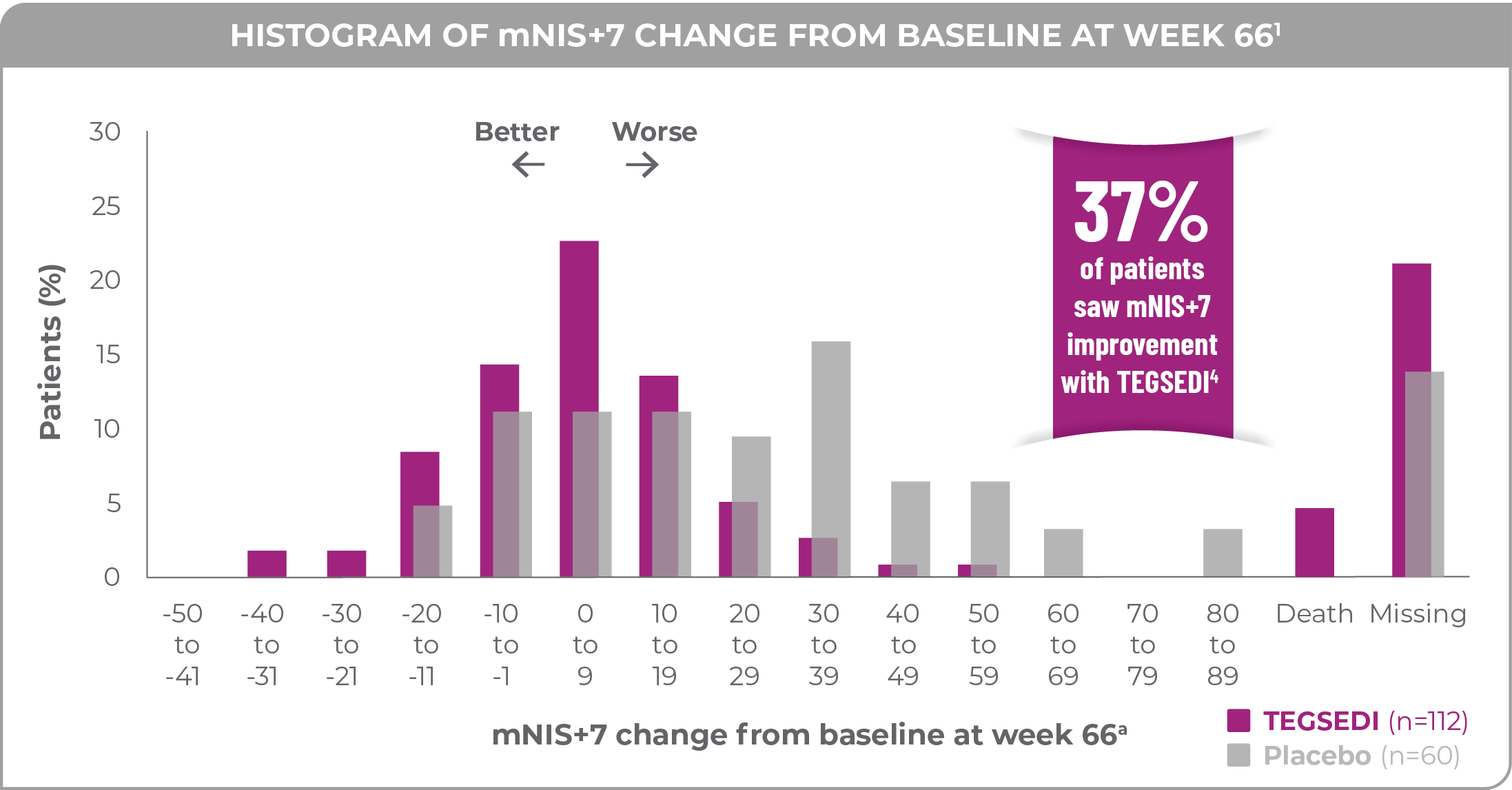
a77% of patients treated with TEGSEDI and 87% of patients receiving placebo completed 66 weeks of the assigned treatment.1
Norfolk QoL-DN results at 66 weeks
Patients treated with TEGSEDI achieved an 11.7-point benefit in Norfolk QoL-DN at 66 weeks vs patients receiving placebo1,2
- Baseline Norfolk QoL-DN was2
- 48.2 ± 27.5 in the TEGSEDI group (n=112)
- 48.7 ± 26.7 in the placebo group (n=60)
- 48.4 ± 27.2 total (n=172)
49% of patients treated with TEGSEDI saw improvement at 66 weeks vs 23% of patients receiving placebo (P=0.008)4

a77% of patients treated with TEGSEDI and 87% of patients receiving placebo completed 66 weeks of the assigned treatment.1
With a weekly injection that can be self-administered at a time of their choosing, patients are empowered to manage the polyneuropathy of hereditary ATTR amyloidosis in the comfort and security of their own homes1

SECONDARY AND TERTIARY ENDPOINTS
NEURO-TTR also assessed secondary and tertiary endpoints
Secondary endpoints
The secondary endpoints of the study were to evaluate TEGSEDI vs placebo based on the change from baseline to week 65 in measures that included4
- Norfolk QoL-DN symptoms domain score in patients with stage 1 polyneuropathy and physical functioning/large fiber neuropathy domain score in patients with stage 2 polyneuropathy
- Serum TTR and retinol binding protein 4 (RBP4)
- BMI and mBMI
- NIS and modified +7
- Neuropathy Impairment Score +7 (NIS+7)
- Global longitudinal strain by echocardiogram
Analysis of secondary endpoints showed a difference in favor of TEGSEDI at 66 weeks for the majority of measures. Certain measures such as mBMI and global longitudinal strain by echocardiogram did not show significance. The analysis of the secondary endpoints were not controlled for Type 1 error and therefore reflect numerical trends only.4
Stage subgroups
Patients treated with TEGSEDI® saw a benefit in neuropathy and QoL regardless of disease stage11
On average, in patients receiving placebo, patients with stage 2 disease experienced a faster progression of neuropathy than those with stage 1 disease11
TTR knockdown
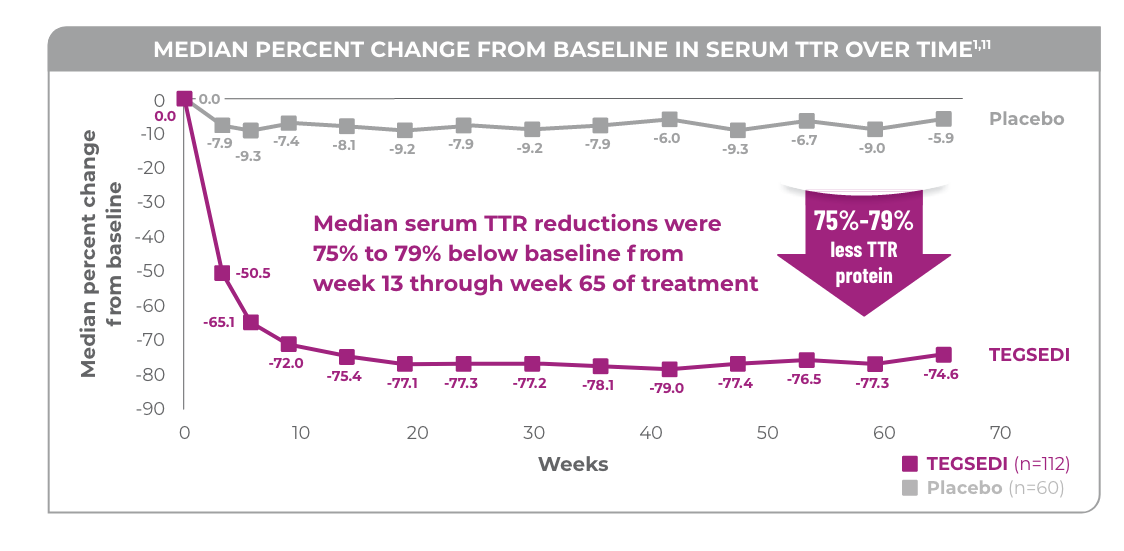
In the NEURO-TTR study, TEGSEDI delivered powerful TTR knockdown (median range: 75% to 79%)1,2
Initiation of TEGSEDI therapy substantially reduced TTR protein levels, and reductions were sustained through week 65 of treatment.1
- In the TEGSEDI group, reductions in circulating TTR reached steady-state levels by week 13 and were sustained through the end of the intervention period2
- Similar TTR reductions were observed regardless of TTR mutation, sex, age, or race1
In patients with the polyneuropathy of hereditary ATTR amyloidosis, TEGSEDI powerfully knocks down (median range: 75% to 79%) circulating TTR protein levels and reduces formation of TTR amyloid fibril deposits1,2
Tertiary endpoint: Short-Form 36 Health Survey (SF-36)
The SF-36 questionnaire is a 36-question, non-disease-specific health survey to assess the participant’s perceived functional health and well-being. It consists of11:
- Physical Component Summary Score (PCS)
- Mental Component Summary Score (MCS)
- Eight individual domain scores; physical function, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, and mental health
At week 65, the placebo group had experienced clinically significant worsening in mean change from baseline in physical component summary (PCS) score4
More patients on placebo experienced substantial impairment as measured by SF-36 physical functioning item responses10
aFigure shows change from baseline to week 66 in percentage of patients in each treatment group whose item response indicated substantial impairment. A patient was considered to have substantial impairment in physical functioning items if they responded they were “limited a lot” in a specific physical function or work, social, or other activity.10
The analysis of the tertiary endpoints were not controlled for Type 1 error and therefore reflect numerical trends only.

Safety
Safety outcomes in NEURO-TTR
Adverse reactions were reported in ≥5% of TEGSEDI-treated patients and ≥5% more frequently or ≥2x more frequently than in patients receiving placebo1
aIncludes bruising, erythema, hematoma, hemorrhage, induration, inflammation, mass, edema, pain, pruritus, rash, swelling, and urticaria.1
bIncludes arrhythmia, atrial fibrillation, atrial flutter, bradyarrhythmia, bradycardia, extrasystoles, sinus arrhythmia, sinus bradycardia, supraventricular extrasystoles, tachycardia, and ventricular extrasystoles.1
cIncludes bacteremia, cellulitis staphylococcal, clostridium difficile infection, conjunctivitis bacterial, cystitis Escherichia, Helicobacter gastritis, Helicobacter infection, and staphylococcal infection.1
-
TEGSEDI can cause serious adverse reactions, including thrombocytopenia and glomerulonephritis1
- Serious adverse reactions were more frequent in patients treated with TEGSEDI (32%) than in patients receiving placebo (21%)1
The mean rate of injection site reactions in the TEGSEDI group was 1.1% of all injections administered2
- 97% of the events were mild in severity and no patients discontinued TEGSEDI prematurely due to injection site reactions2
- Injection site reactions occurred in 49% of patients treated with TEGSEDI compared with 10% of patients receiving placebo1
77% of patients treated with TEGSEDI and 87% of patients receiving placebo completed 66 weeks of the assigned treatment
No new safety signals were reported in the open-label extension3
Platelet and renal monitoring implemented in the NEURO-TTR study was successful in identifying patients at risk of glomerulonephritis and thrombocytopenia3
- There were no cases of grade 4 platelet count decrease or glomerulonephritis in the open-label extension study3
- Discontinuation because of adverse events occurred in 15 patients (18%) in the TEGSEDI-TEGSEDI group and 4 patients (8%) in the placebo-TEGSEDI group3
- Serious treatment-emergent adverse events occurred in 33 patients (39%) and 14 patients (28%) in the TEGSEDI-TEGSEDI and placebo-TEGSEDI groups, respectively, and were considered related to treatment in 4 patients (5%) and 1 patient (2%), respectively3
- 9 fatal adverse events occurred during the open-label extension study, none were considered related to treatment3
No new safety signals were identified in the 2-year interim analysis of the open-label extension study of the patients from the NEURO-TTR population.3
The most common (≥10%) adverse events across both treatment groups were3
- Nausea
- Urinary tract infection
- Vomiting
- Diarrhea
- Fatigue
- Chills
- Falls
- Peripheral edema
- Injection site pain
- Thrombocytopenia
- Syncope
- Injection site erythema
- Headache
- Muscular weakness
- Myalgia
- Dyspnea
There were no cases of grade 4 platelet count decrease or glomerulonephritis in the open-label extension study.3
Platelet and renal monitoring were shown to be effective in the open-label extension study3,4

Open-label extension study
97% (135/139) of patients who completed NEURO-TTR elected to continue on to the open-label extension study3
- At the conclusion of the NEURO-TTR study, patients were given the opportunity to enroll in an open-label extension study and continue on treatment with TEGSEDI or switch from placebo3
- Enrollment in the open-label extension study was generally limited to only those patients who met eligibility criteria, completed NEURO-TTR, and elected to enroll in the open-label extension study3
- The open-label extension (OLE) study is ongoing and the data presented here are based on an interim analysis at 2 years when 44% of patients enrolled in OLE had completed week 104 during the treatment period3
- No statistical analyses were performed on the OLE data and thus the results are only qualitative3
- The open-label extension study was not a placebo-controlled study and all patients in the open-label extension study were aware they were on active drug
- 50 patients have had more than 3 years of exposure to TEGSEDI from the NEURO-TTR study and open-label extension4
- Mean duration in the same data set was 847.2 days, and median duration of exposure was 910.5 days4
- The primary objective of the OLE study was to assess safety and it was consistent with the pivotal study
Open-label extension study results
Patients treated with TEGSEDI saw less progression of neuropathy3
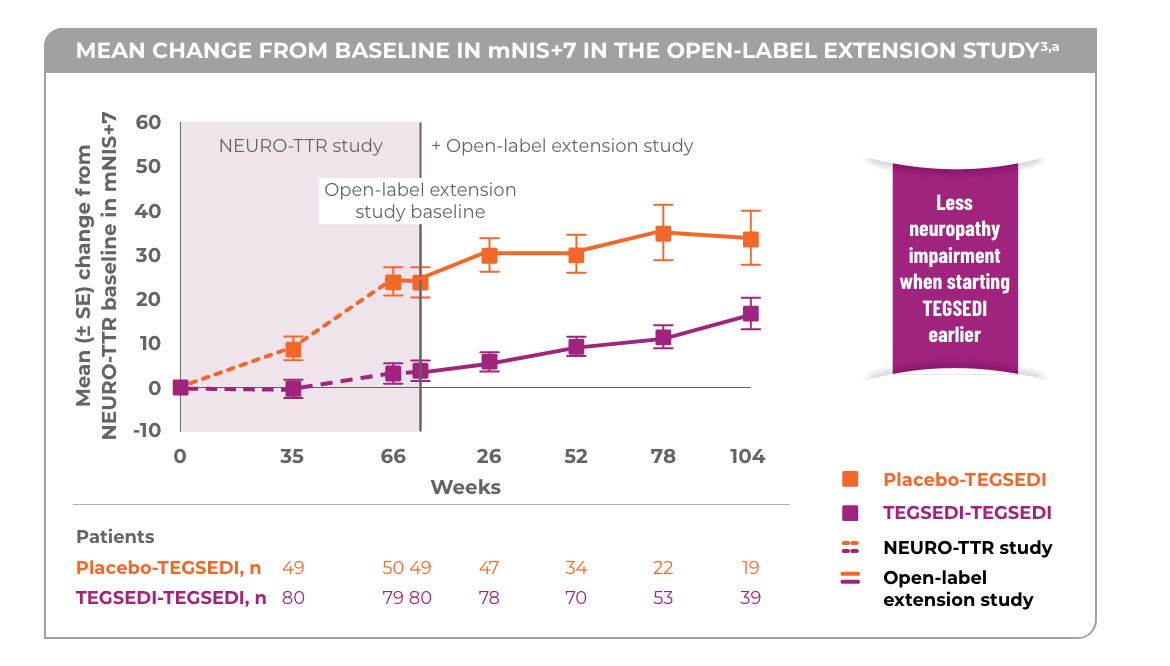
aNo statistical analyses were performed and thus the results are only qualitative.3
Patients treated with TEGSEDI® saw a benefit in QoL vs placebo in the open-label extension
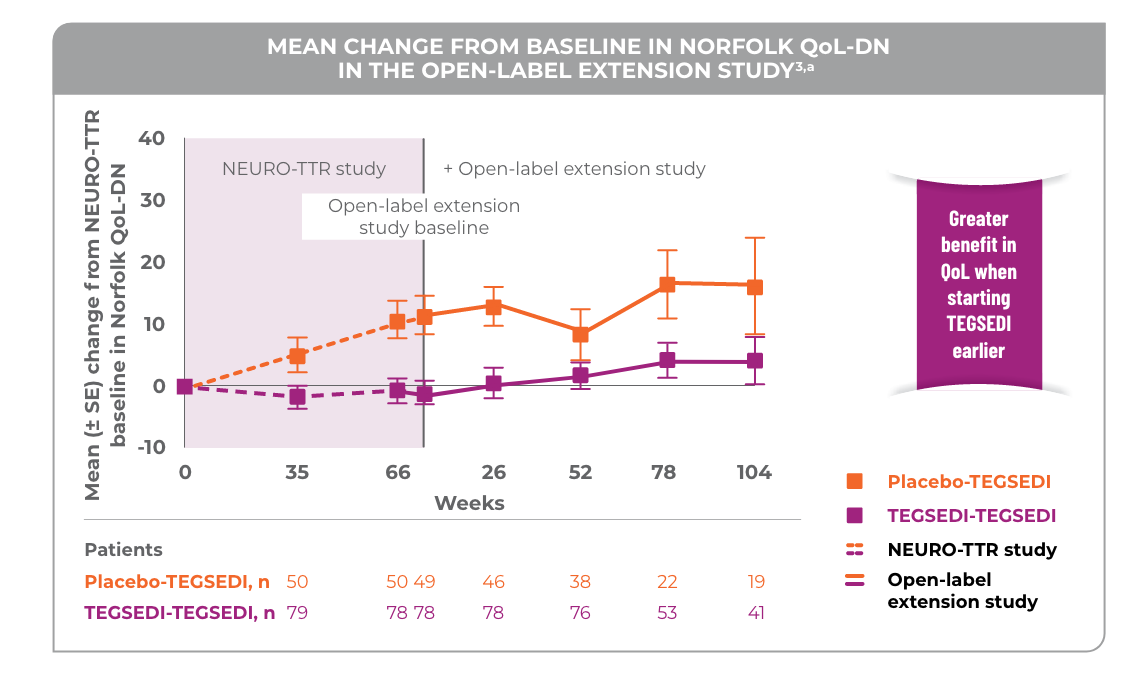
aNo statistical analyses were performed and thus the results are only qualitative.3
Patients who continued on treatment with TEGSEDI from the pivotal study saw a benefit in SF-36 PCS Score3
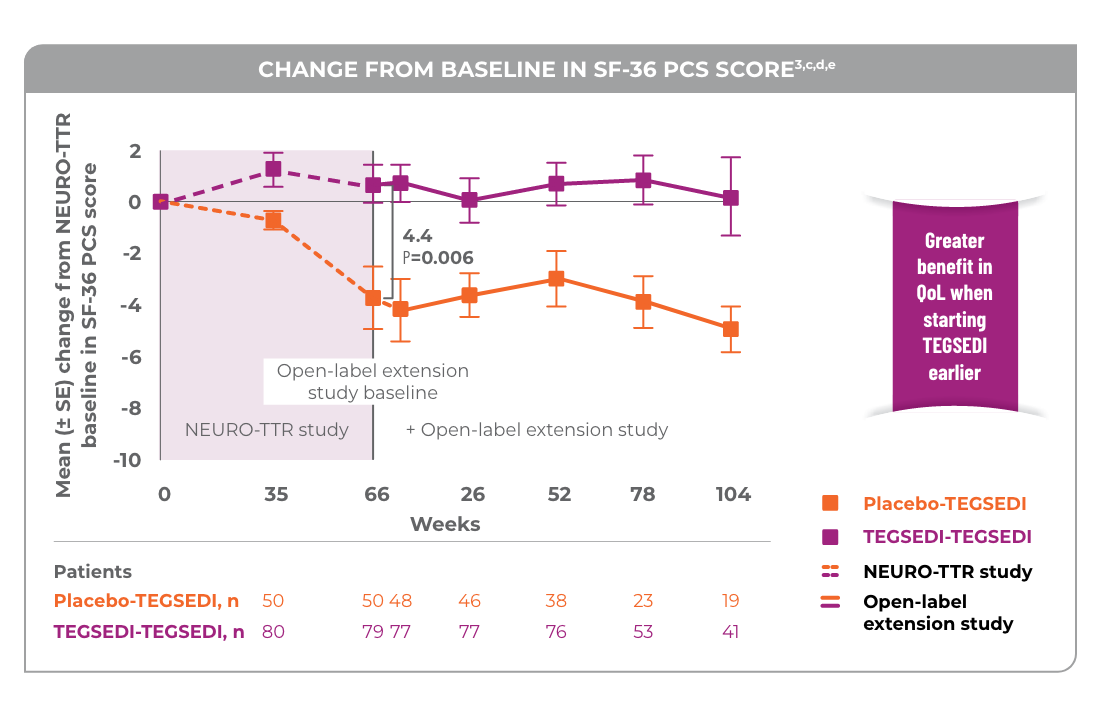
cNo statistical analyses were performed and thus the result are only qualitative.3
dThe pivotal study and open-label extension study used SF-36v2.3
eA greater value indicates a greater QoL.4
Patients who switched from placebo to TEGSEDI saw a benefit in measures of neuropathy and QoL compared with a continued predicted worsening with placebo3


Image is not of actual patients
When he was able to get on TEGSEDI, we started seeing him feeling better already and it was a hallelujah moment for our family. We felt so lucky.
Quote taken from the caregiver of an actual patient taking TEGSEDI


Image is not of actual patients
You’ve given me my quality of life back—you’ve given my family their father, their brother, their grandpa back, and in a good mood. That wouldn’t happen without TEGSEDI.
Quote taken from an actual patient taking TEGSEDI